TEM is mainly applied to study the
inside of -sections of- very small object, e.g. membranes, nuclei and
organelles of cells. But also extremely thin structures as a whole, like
virussen and macromolecular aggregates (e.g.
rings of porphyrin polymers), can be investigated with this instrument. The blackening in the final image is determined by the electron opacity of the object, which depends on its local thickness and the atomic composition of the material. A TEM provides thus different information than a scanning electron microscope (SEM) that produces images that tell something about the topography (surface) of the sample.
| Examples of TEM images |
 |
![[Negatief gekleurde gezuiverde Cowpea Chlorotic Mottle Virus (CCMV)] [negatively stained purified Cowpea Chlorotic Mottle Virus (CCMV)]](/images/virus01_small.jpg) |
![[Negatief gekleurde gezuiverde Cowpea Chlorotic Mottle Virus (CCMV)] [negatively stained purified Cowpea Chlorotic Mottle Virus (CCMV)]](/images/virus02_small.jpg) |
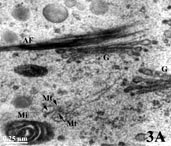
Organelles in a pollen grain of tobacco (Nicotiana tabacum; AF = Actin filaments; G = Golgi apparatus; Mi = Mitochondrion; Mt = Microtubule; zoom).
Imaging: A.M. Wolters-Arts, Radboud University Nijmegen). |
Hexagonal pattern purified Cowpea Chlorotic Mottle Virusses (CCMV) negatively stained with uranyl acetate (purification under high salt concentration; zoomWork of Marta Comellas (Organic Chemistry) | Random pattern of CCMV, this time purified in a lower salt concentration (zoom)
Work of Marta Comellas (Organic Chemistry) |
preparation of the samples
Untreated biological material is usually too thick to allow electrons to pass through. Therefore the samples are nearly always cut in advance in ultrathinsections of about 70 nm. If the sections would be thicker all electrons would be shielded off and the image would be totally black. Ultrathick sectioning is done with an ultramicrotome and special glass or diamond knifes. This preparation required precision skills and a lot of experience.
Raw biological material shows little differences in electron opacity, so that without pre-treatment a merely contrasted image would arise in the microscope. To overcome this problem, the material is previously exposed to electron-opaque substances containing
heavy metals, i.e. osmium, that bind specifically to some structures in the sample. In the TEM picture, these 'metal-stained' structures appear then as dark parts. On the other side, other structures with less affinity for those 'stains' will transmit more electrons and show up as clear shapes on the screen.
The viewer here below shows pictures of the preparation of samples for TEM observation:
- Fixation: Parts of raw material, e.g. a piece of biological tissue of a few millimeters or single cells, are fixed with chemical products (e.g. glutaraldehyde) or cold (liquid propane at - 90°C, for physical fixation). Fixation is done to preserve the fine structure in the cells in a state so close as possible as to the living state and to make the cells permeable for the components required in the next steps of preparation. Physical fixatives and chemical fixatives and most other chemicals employed during sample preparation for TEM are either dangerously cold or toxic. Therefore protection through a fume hood, a labcoat, gloves and safety glasses is a must.
- Rinsing and 'staining': After fixation and rinsing away of the fixative, the material is treated with heavy metal compounds (for example osmiumtetroxide, uranylacetate or potassium permanganate) that bind preferably to certain regions like lipid-rich membranes of organelles, DNA-clusters in the cell nucleus, and protein-rich structures like cytoskeletal elements. Such metal-compounds have the property to reflect electrons so that labeled structures appear as dark areas in the electron microscope view. This processing step is also called staining although true colors are not employed in electron microscopy. In the TEM-image example showing a portion of cytoplasm in a tobacco pollen tube with membranes of a mitochondrion [= Mi] and two Golgi units [G], as well as protein-rich microtubuli [Mt] and actin filaments [AF] (both cytoskeletal elements) 'stained' black.
- Dehydratation: To be able to cut extremely thin sections (of 60-90 nm thin) that keep their consistency and yet allow electrons to pass the stained samples are imbedded in resins (plastics). Because most of these resins are hydrophofic, it is necessary to remove all water from the sample through wash steps of increasing ethanol or aceton concentration, followed by final washes in another apolar substance like propylene oxide.
- Imbedding in resin: Then the material is gradually infiltrated with the still unpolymerized resin (e.g. methacrylates, polyester and epoxy-resins, acryls, polyethyleneglycol and others) solved in the apolar and volatile transition medium that evaporates. The concentration of resins raises until saturation and the sample gets a viscuous consistence. Little pieces of resin-infiltrated material are placed in small holders, e.g. gelatin capsule like used for medical drugs; the remaining volume in the holder is filled with fresh resin. Then, a polymerisation treatment follows induced by heat from an oven or with amicro-wave or by catalytic reaction with ultraviolet light, so that hard and easily sectionable blocks of resin and material are obtained.
- Trimming of the block of resin and ultrathin sectioning: sections with a thickness of about 70 nm can only be cut with special knifes of cleeved glass of high purity made with a glass-knife maker (which breaks glass in triangles with extremely sharp edges) or with a diamond knife. The cutting is done with a ultra-microtome (a precision cutting instrument) with numerous setting possibilities. After each section the microtome sampleholder shifts exactly one step of about 70 nm furtherup toward the knife. The mechanical resistance during sectioning is held as low by making the section surface as small as possible; therefore the block of resin with material has been previously trimmed to a piramidal-shaped stub with a flat top. The topsurface itself has a trapezium-shape and a size of about one by a half millimeter (see here three magnifications of a trimmed block of resin with inbedded pollen grains: low,
medium, high).
- Picking up sections on a grid: Water in a tiny container behind the knife contributes to lubrification of the cut edge. Sections float on that surface (in air they would easily become electrostatic and jump aside or be blown away). The thickness of the section can be estimated with quite high accuracy from the reflection color of the floating section. These very fragile rows of trapezoid-shaped sections can be lift up like with a spoon from the water surface onto a so-called grid. A grid is a metallic disc with a grid pattern that has been previously covered by an electron-translucent sheet of Formvar that functions like a support for the sections. Grids are later inserted into the electron microscope with help of a rod-shaped holder. (Note: only sections that fall between the bars of the grid can be observed later on).