Porphyrin rings |
|||||||||||||
Porphyrin rings: self-assembling catalyst molecules
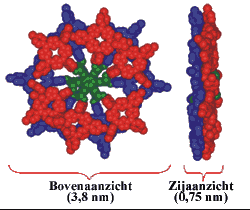
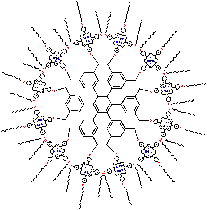
In nature, chemical reactions are catalysed by several enzymes. (A catalyst is a substance that accelerates a chemical reaction without being consumed in the process). Those catalytic enzymes often employ porphyrins in the active site for the catalytic function. One example is the enzyme Cytochrome P-450 that can catalyse oxidation reactions. In the laboratory for Organic Chemistry (Radboud University Nijmegen) artificial, bio-inspired catalysts are developed that also consist of porphyrins. Each molecule contains 12 porphyrins (a dodecamer) that are bound to a central core molecule. These giant molecules have the shape of a disc (diameter: 38 Å = 3.8 nm) and the molecular weight (15 kDa) is comparable to that of small proteins.
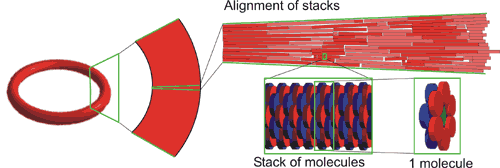
By their specific design, these porphyrin dodecamers have self-assembling properties. On carbon-coated copper grids these molecules form micro-meter sized, stable, solid rings. Supramolecular interactions between the porphyrins (so-called pi-pi-stacking) are the driving force to form these structures. The rings were imaged and investigated by transmission (TEM)- and scanning electron microscopy (SEM). Besides, the precise orientation of the individual molecules within the rings was determined by applying polarized fluorescence microscopy (Pol-Fluo); the different colors of the emitted light correlate with the orientation of the molecules. The aggregation of the molecules into stacks has been visualized by scanning tunneling microscopy (STM; surf to the Scanning Probe Microscope). (Research project of Marga Lensen) Since the interior of the rings is empty, nanotechnology researchers of the Radboud University Nijmegen intend to use these structures as containers. They want to fill the rings with substrate solutions and perform catalysis on the surface using the porphyrins in the rings as catalysts. The catalytic reaction of interest is the epoxidation (a chemical reaction in which an oxygen atom is inserted in the double bond of an alkene) that the enzyme Cytochrome P-450 catalyses in nature. Cytochrome P-450 is found in human in the liver, where it is responsible for the conversion of toxic compounds into harmless substances.
|
|||||||||||||